Direct sp3 C–H acroleination of N-aryl-tetrahydroisoquinolines by merging photoredox catalysis with nucleophilic catalysis†
Abstract
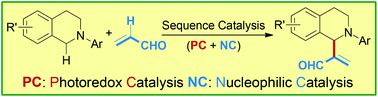
Sequence catalysis merging photoredox catalysis (PC) and nucleophilic catalysis (NC) has been realized for the direct sp3 C–H acroleination of N-aryl-tetrahydroisoquinoline (THIQ). The reaction was performed under very mild conditions and afforded products in 50–91% yields. A catalytic asymmetric variant was proved to be successful with moderate enantioselectivities (up to 83 : 17 er).

 Please wait while we load your content...
Please wait while we load your content...