A new modified cytosine base capable of base pairing with guanine using four hydrogen bonds†
Abstract
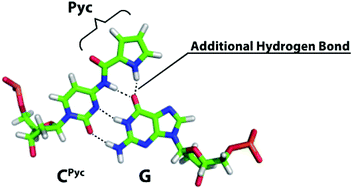
Oligonucleotides, containing 4-N-(1H-pyrrol-2-ylcarbonyl)deoxycytidine (dCPyc) and related derivatives, were synthesized via deprotection using 1.5 M NaOMe/MeOH. Among them, oligodeoxynucleotides containing dCPyc exhibited a higher hybridization affinity for DNA and RNA than the unmodified oligodeoxynucleotides. Comparative analysis between dCPyc and its derivatives by molecular dynamic simulation indicated that the CPyc residue could form four hydrogen bonds with the opposite G nucleobase keeping a more planar structure than the CInc residue where the Pyc group was replaced with a 1H-indol-2-ylcarbonyl group.

 Please wait while we load your content...
Please wait while we load your content...