Cation⋯π interaction and microhydration effects in complexes formed by pyrrolidinium cation and aromatic species in amino acid side chains†
Abstract
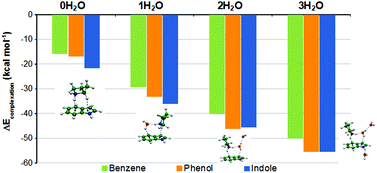
A computational study has been carried out in complexes formed by pyrrolidinium cation and aromatic units present in amino acid side chains. The interaction is stronger with indole (−21.9 kcal mol−1 at the CCSD(T) complete basis set level) than with phenol (−17.4 kcal mol−1) or benzene (−16.1 kcal mol−1). Most stable structures show a N–H⋯π contact between pyrrolidinium cation and the phenyl ring of the three aromatic species, except in phenol complexes where the most stable minimum shows a N–H⋯O hydrogen bond. In phenol and indole complexes, secondary contacts are established between the C–H groups of the carbon skeleton of pyrrolidinium and the aromatic rings or hydroxyl oxygen, being the main reason for the enhanced stability with respect to benzene, where these contacts are not possible. The interaction is mainly controlled by electrostatics, but contributions from induction and dispersion are also significant, especially the latter in indole complexes. These three attractive contributions increase their intensity when going from benzene to phenol and indole. Microhydration effects have been estimated by including up to three water molecules in the complexes. In monohydrated pyrrolidinium⋯benzene complex the most stable structure shows the water molecule coordinated to the cation without interacting with the ring. In phenol and indole, otherwise, the water molecule interacts with both the cation and the aromatic species, forming a cyclic hydrogen bond pattern π(phenyl)⋯H–N–H⋯O–H⋯X (X = π, O). This pattern is also present among the most stable structures found for complexes with two and three water molecules, though a variety of almost isoenergetic minima showing different hydrogen bond patterns have been found. Water molecules remove the stability differences between phenol and indole complexes, which already with two water molecules show similar stabilities, though around 5 kcal mol−1 larger than benzene ones.

 Please wait while we load your content...
Please wait while we load your content...