Copper-catalyzed annulation of α-substituted diazoacetates with 2-ethynylanilines: the direct synthesis of C2-functionalized indoles†
Abstract
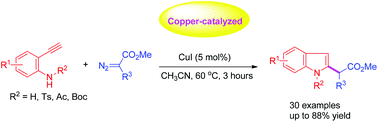
Copper-catalyzed direct annulation of α-substituted diazoacetates with 2-ethynylanilines leading to C2-functionalized indoles was achieved under mild reaction conditions. The C2-(carboxylate methyl) substituted indoles were obtained in moderate to high yields. In addition, this procedure tolerates a series of N-substituted and free substituted 2-ethynylanilines.

 Please wait while we load your content...
Please wait while we load your content...