Platinum-catalyzed anti-stereocontrolled ring-opening of oxabicyclic alkenes with Grignard reagents†
Abstract
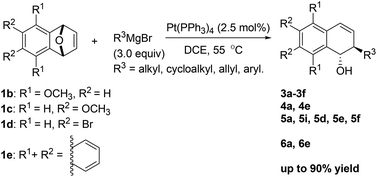
A new platinum-catalyzed anti-stereocontrolled ring-opening of oxabicyclic alkenes with various Grignard reagents was reported, which afforded the corresponding anti-2-substituted-1,2-dihydronaphthalen-1-ol products with moderate to good yields in the presence of a catalytic amount of Pt(PPh3)4 (2.5 mol%) under mild conditions. The effects of catalyst loading, solvent and temperature on the yield were also investigated. Furthermore, the trans-configuration of the product 5i was confirmed by X-ray diffraction analysis.

 Please wait while we load your content...
Please wait while we load your content...