Design, synthesis, and biological evaluation of novel trifluoromethyl indoles as potent HIV-1 NNRTIs with an improved drug resistance profile†
Abstract
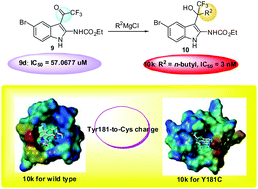
A novel series of trifluoromethyl indole derivatives have been designed, synthesized and evaluated for anti-HIV-1 activities in MT-2 cells. The hydrophobic constant, acute toxicity, carcinogenicity and mutagenicity were predicted. Trifluoromethyl indoles 10i and 10k showed extremely promising activities against WT HIV-1 with IC50 values at the low nanomolar level, similar to efavirenz, better than nevirapine, and also possessed higher potency towards the drug-resistant mutant strain Y181C than nevirapine. Preliminary SAR and docking studies of detailed binding mode provided some insights for discovery of more potent NNRTIs.

 Please wait while we load your content...
Please wait while we load your content...