A density functional study of chiral phosphoric acid-catalyzed direct arylation of trifluoromethyl ketone and diarylation of methyl ketone: reaction mechanism and the important role of the CF3 group†
Abstract
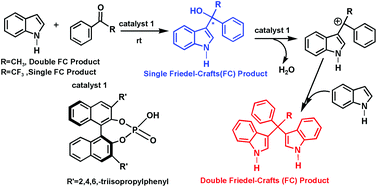
The detailed mechanism of the chiral phosphoric acid-catalyzed diarylation reaction between acetophenone and indole has been investigated by DFT methods and compared with that of the reaction between 2,2,2-trifluoroacetophenone and indole. The calculated results confirm our previous hypothesis that the CF3 group in the ketone plays a perfect double role in activating the substrate and stabilizing the single arylation product of tertiary alcohol. It is also demonstrated that the different ratio of the F-substitution in the CH3 group of methyl ketone (CH3−nFn, n = 0, 1, 2, 3) affects the activation energy of the key dehydration step for the proposed diarylation process differently, and determines whether the subsequent re-arylation proceeds or is being suppressed. The computational prediction that the prohibitive barriers for CF3 and CHF2 ketones in the rate-determining dehydration step for the diarylation process could be overcome at higher reaction temperature has been validated by our additional experiments at 80 °C. Furthermore, the origin of the high enantioselectivity of the chiral phosphoric acid-catalyzed single arylation of trifluoromethyl ketone has been studied with the two-layer ONIOM method. The experimentally observed enantiomeric excess can be successfully rationalized.

 Please wait while we load your content...
Please wait while we load your content...