Rhodium-catalyzed intermolecular hydroarylation of internal alkynes with N-1-phenylbenzotriazoles†
Abstract
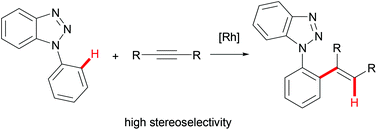
A rhodium-catalyzed intermolecular hydroarylation of internal alkynes with N-1-phenylbenzotriazoles via C–H bond activation is described. This transformation offers an alternative method for the hydroarylation of internal alkynes with high stereoselectivity. The reaction mechanism was discussed according to the deuterium-labeling experiments.

 Please wait while we load your content...
Please wait while we load your content...