Microwave assisted base dependent regioselective synthesis of partially reduced chromenes, isochromenes and phenanthrenes†‡
Abstract
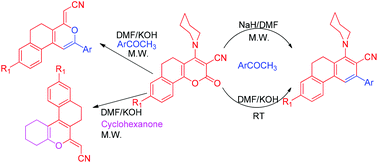
We have reported a microwave assisted base directed regioselective synthesis of partially reduced chromenes, isochromenes and phenanthrenes. Functionalized 4-(piperidin-1-yl)-5,6-dihydro-2H-benzo[h]-chromen-2-one-3-carbonitriles have been used as precursors, which on reaction with functionalized acetophenones in the presence of KOH in DMF under microwave irradiation yield (Z)-2-(2-aryl-5,6-dihydro-4H-benzo[f]isochromen-4-ylidene)acetonitriles. The use of NaH in DMF provides 3-aryl-1-(piperidin-1-yl)-9,10-dihydro phenanthrene-2-carbonitriles in excellent yield regioselectively. The use of cyclohexanone as a nucleophile source yields (Z)-2-(3,4,7,8-tetrahydro-1H-naphtho[2,1-c]chromen-6(2H)-ylidene)acetonitriles. The structure and geometry of isochromene have been proved without any ambiguity by single crystal X-ray diffraction.

 Please wait while we load your content...
Please wait while we load your content...