The acid-catalysed synthesis of 7-azaindoles from 3-alkynyl-2-aminopyridines and their antimicrobial activity†
Abstract
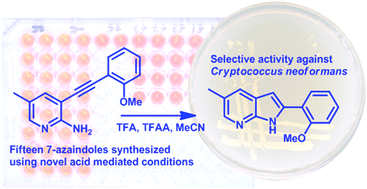
The synthesis of 7-azaindoles from 3-alkynyl-2-aminopyridines using acidic conditions, namely, a mixture of trifluoroacetic acid (TFA) and trifluoroacetic anhydride (TFAA), is described. This methodology resulted in the synthesis of fifteen 7-azaindoles, with most containing substituents at the 2- and 5-positions. The majority of these were tested for antimicrobial activity against a range of bacteria and yeasts. The 7-azaindoles displayed the best activity against the yeasts, particularly against Cryptococcus neoformans, where activities as low as 3.9 μg ml−1 were observed.

 Please wait while we load your content...
Please wait while we load your content...