Hydrophilic tetracarboxy bacteriochlorins for photonics applications†
Abstract
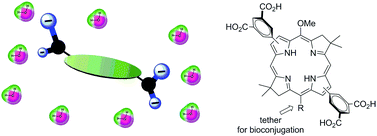
Bacteriochlorins absorb strongly in the near-infrared (NIR, 700–900 nm) region and hence are well suited for photophysical studies and photomedical applications, yet such endeavors heretofore have been largely limited by the intrinsic lipophilicity of the bacteriochlorin macrocycle. Here, a new molecular design is investigated wherein 3,5-dicarboxyphenyl units are appended to the β-pyrrolic positions of the bacteriochlorin. Use of the 3,5-aryl substitution motif places the carboxylic acid groups, which are anionic at neutral pH, above and below the plane of the bacteriochlorin macrocycle. A de novo synthesis has been employed to create five such bacteriochlorins, which uses as intermediates two new 2,12-dibromobacteriochlorin building blocks and a known 3,13-dibromobacteriochlorin. The aryl groups with protected carboxylate moieties were introduced by Suzuki coupling; subsequent deprotection afforded the hydrophilic bacteriochlorins. The latter were characterized by absorption and fluorescence spectroscopy in DMF and in aqueous phosphate buffer (pH 7). In most cases, comparable sharp emission (FWHM of ∼25 nm) and modest fluorescence yields (0.060–0.11) were observed in aqueous phosphate buffer medium and in DMF. Aqueous solubility was examined by absorption spectral interrogation of samples over a 1000-fold concentration range with reciprocal change in pathlength (∼0.5, 5, 50, and 500 μM; 10, 1, 0.1, and 0.01 cm pathlength cuvettes). One hydrophilic bacteriochlorin was prepared that contains a single maleimido-terminated tether for bioconjugation; the tether was installed by the sequence of 15-bromination of the bacteriochlorin, Suzuki coupling, and DCC-mediated amide formation. The maleimido-bacteriochlorin was conjugated to a 48-residue cysteine-containing peptide analogue of a constituent from a bacterial photosynthetic light-harvesting complex. Taken together, the results show a new molecular design and facile de novo synthetic route for obtaining hydrophilic bacteriochlorins including a bioconjugatable group if desired.

 Please wait while we load your content...
Please wait while we load your content...