DABCO catalyzed cross-Rauhut–Currier/transesterification reactions of activated alkenes with phenyl acrylates: scope and mechanistic insight†
Abstract
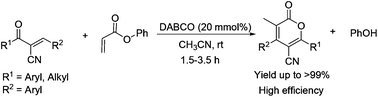
DABCO catalyzed the cross-Rauhut–Currier/transesterification reaction of α-cyano-α,β-unsaturated ketones and aryl acrylates was discovered. The reaction rate law was determined by an integral method under pseudo-first-order reaction conditions, which assisted in proposing the mechanism of cross-Rauhut–Currier reaction promoted by Brønsted acid and establishing the rate-determining step.

 Please wait while we load your content...
Please wait while we load your content...