BixLa1−xVO4 solid solutions: tuning of electronic properties via stoichiometry modifications†
Abstract
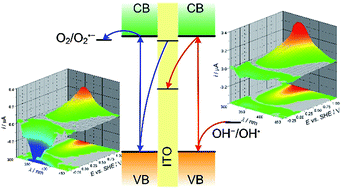
BixLa1−xVO4 solid solutions were obtained in the form of fine powder via a microwave-assisted hydrothermal route. The presence of a solid solution in the studied system was confirmed using X-ray diffraction (XRD) and optical spectroscopy techniques. Pure BiVO4 and LaVO4 were obtained in the monoclinic form, whereas solid solutions in the tetragonal, zircon-type structure. The optical band gap dependence on the composition of the solid solution is parabolic, thus there is a possibility to tune this parameter in a wide concentration range, from 2.4 to 4.0 eV. An absorption coefficient maximum is also concentration-dependent, possibly, due to the structural disorder of the samples. Solid solutions with Bi3+ concentration between 11.94 and 32.57 at.% exhibit intense, green luminescence. This indicates the presence of Bi-originated electronic states within the band gap. The value of the conduction band edge potential, measured by both electrochemical impedance spectroscopy and work function measurements, is concentration-independent. Moreover, solid solutions exhibit a photoelectrochemical photocurrent switching effect, thus they may be promising materials for molecular electronics and as dioxygen activators.

 Please wait while we load your content...
Please wait while we load your content...