Synthesis of Cu, Zn and Cu/Zn brass alloy nanoparticles from metal amidinate precursors in ionic liquids or propylene carbonate with relevance to methanol synthesis†
Abstract
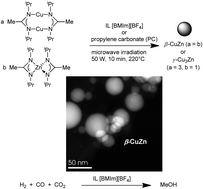
Microwave-induced decomposition of the transition metal amidinates {[Me(C(NiPr)2)]Cu}2 (1) and [Me(C(NiPr)2)]2Zn (2) in the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIm][BF4]) or in propylene carbonate (PC) gives copper and zinc nanoparticles which are stable in the absence of capping ligands (surfactants) for more than six weeks. Co-decomposition of 1 and 2 yields the intermetallic nano-brass phases β-CuZn and γ-Cu3Zn depending on the chosen molar ratios of the precursors. Nanoparticles were characterized by high-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM), dynamic light scattering and powder X-ray diffractometry. Microstructure characterizations were complemented by STEM with spatially resolved energy-dispersive X-ray spectrometry and X-ray photoelectron spectroscopy. Synthesis in ILs yields significantly smaller nanoparticles than in PC. β-CuZn alloy nanoparticles are precursors to catalysts for methanol synthesis from the synthesis gas H2/CO/CO2 with a productivity of 10.7 mol(MeOH) (kg(Cu) h)−1.

 Please wait while we load your content...
Please wait while we load your content...