Water oxidation electrocatalysis by a zeolitic imidazolate framework†
Abstract
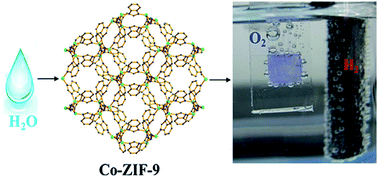
The search for efficient water oxidation catalysts (WOCs) is of paramount importance in energy and environmental fields, but there exists no good non-noble catalyst that works under acidic and alkaline conditions. Intensive investigations have recently focused on cobalt based complex/solid catalysts. Here, we have introduced a new type of cobalt-based WOC made of metal–organic frameworks where the redox function of cobalt centres was modified by imidazolate linkers for facilitating the proton transfer process. This cobalt-containing zeolitic imidazolate framework (Co-ZIF-9) has been demonstrated for the first time to electrocatalyze the oxygen evolution reaction in a wide pH range. The catalyst was found by theoretical calculation to be capable of activating the water molecule via binding the OH-group to the metal sites with low activation barriers, while the eliminated proton was accepted by the nearby benzimidazolate motifs. This allows Co-ZIF-9 to work effectively for the electrochemical oxygen-evolution reaction.

 Please wait while we load your content...
Please wait while we load your content...