Environmentally responsive histidine–carboxylate zipper formation between proteins and nanoparticles†
Abstract
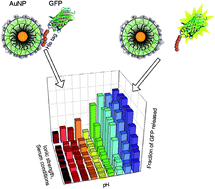
Interfacing synthetic materials with biomacromolecules provides new systems for biological applications. We report the creation of a reversible multivalent supramolecular “zipper” recognition motif between gold nanoparticles and proteins. In this assembly, carboxylate-functionalized nanoparticles interact strongly with oligohistidine tags. This interaction can be tuned through His-tag length, and offers unique binding profiles based on the pH and electrolyte concentration of the medium.

 Please wait while we load your content...
Please wait while we load your content...