Molybdenum disulfide/pyrolytic carbon hybrid electrodes for scalable hydrogen evolution†
Abstract
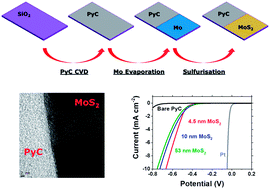
The electrochemical generation of hydrogen fuel via the proton reduction in the Hydrogen Evolution Reaction (HER) in aqueous media is currently dependent on the use expensive noble metal catalysts for which alternatives must be sought. Molybdenum disulfide (MoS2) has shown great promise as a suitable electrocatalyst in this regard. While many lab-scale experiments on the HER activity of this material have demonstrated its viability and explored some fundamental mechanistic features of HER at MoS2, these experimental techniques are often ill-suited to large scale production of such electrodes. In this study we present work on the fabrication of MoS2/pyrolytic carbon (PyC) electrodes via vapour phase sulfurization of Mo thin films. These hybrid electrodes combine the catalytic activity of MoS2 with the conductivity and stability of PyC, whilst using industrially compatible processing techniques. Structural defects in the sulfur lattice were found to be key catalytically active sites for HER and thinner MoS2 films displayed a higher quantity of these defects and, hence, an improved HER activity. The observed Tafel slope of 95 mV decade−1 is comparable to previous literature works on MoS2 HER performance.

 Please wait while we load your content...
Please wait while we load your content...