The Aβ peptide forms non-amyloid fibrils in the presence of carbon nanotubes†
Abstract
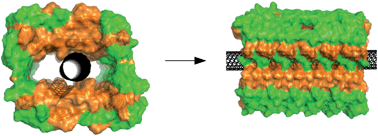
Carbon nanotubes have specific properties that make them potentially useful in biomedicine and biotechnology. However, carbon nanotubes may themselves be toxic, making it imperative to understand how carbon nanotubes interact with biomolecules such as proteins. Here, we used NMR, CD, and ThT/fluorescence spectroscopy together with AFM imaging to study pH-dependent molecular interactions between single walled carbon nanotubes (SWNTs) and the amyloid-beta (Aβ) peptide. The aggregation of the Aβ peptide, first into oligomers and later into amyloid fibrils, is considered to be the toxic mechanism behind Alzheimer's disease. We found that SWNTs direct the Aβ peptides to form a new class of β-sheet-rich yet non-amyloid fibrils.

 Please wait while we load your content...
Please wait while we load your content...