Redox cycling in nanoporous electrochemical devices†
Abstract
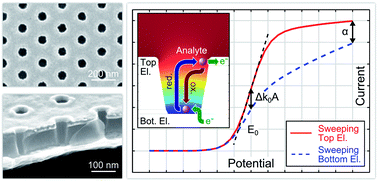
Nanoscale redox cycling is a powerful technique for detecting electrochemically active molecules, based on fast repetitive oxidation and reduction reactions. An ideal implementation of redox cycling sensors can be realized by nanoporous dual-electrode systems in easily accessible and scalable geometries. Here, we introduce a multi-electrode array device with highly efficient nanoporous redox cycling sensors. Each of the sensors holds up to 209 000 well defined nanopores with minimal pore radii of less than 40 nm and an electrode separation of ∼100 nm. We demonstrate the efficiency of the nanopore array by screening a large concentration range over three orders of magnitude with area-specific sensitivities of up to 81.0 mA (cm−2 mM−1) for the redox-active probe ferrocene dimethanol. Furthermore, due to the specific geometry of the material, reaction kinetics has a unique potential-dependent impact on the signal characteristics. As a result, redox cycling experiments in the nanoporous structure allow studies on heterogeneous electron transfer reactions revealing a surprisingly asymmetric transfer coefficient.

 Please wait while we load your content...
Please wait while we load your content...