Natural product and natural product derived drugs in clinical trials†
Abstract
Covering: 2008–2013. Previous review: 2008, 25, 475
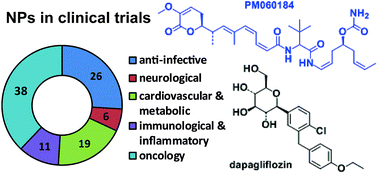
There are a significant number of natural product (NP) drugs in development. We review the 100 NP and NP-derived compounds and 33 Antibody Drug Conjugates (ADCs) with a NP-derived cytotoxic component being evaluated in clinical trials or in registration at the end of 2013. 38 of these compounds and 33 ADCs are being investigated as potential oncology treatments, 26 as anti-infectives, 19 for the treatment of cardiovascular and metabolic diseases, 11 for inflammatory and related diseases and 6 for neurology. There was a spread of the NP and NP-derived compounds through the different development phases (17 in phase I, 52 in phase II, 23 in phase III and 8 NDA and/or MAA filed), while there were 23 ADCs in phase I and 10 in phase II. 50 of these 100 compounds were either NPs or semi-synthetic (SS) NPs, which indicated the original NP still plays an important role. NP and NP-derived compounds for which clinical trials have been halted or discontinued since 2008 are listed in the Supplementary Information. The 25 NP and NP-derived drugs launched since 2008 are also reviewed, and late stage development candidates and new NP drug pharmacophores analysed. The short term prospect for new NP and NP-derived drug approvals is bright, with 31 compounds in phase III or in registration, which should ensure a steady stream of approvals for at least the next five years. However, there could be future issues for new drug types as only five new drug pharmacophores discovered in the last 15 years are currently being evaluated in clinical trials. The next few years will be critical for NP-driven lead discovery, and a concerted effort is required to identify new biologically active pharmacophores and to progress these and existing compounds through pre-clinical drug development into clinical trials.

 Please wait while we load your content...
Please wait while we load your content...