Hollow carbon nanofibers as an effective electrode for brackish water desalination using the capacitive deionization process
Abstract
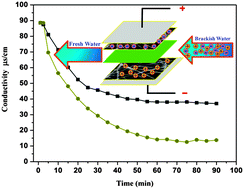
Capacitive deionization (CDI) is strongly recommended as an environmentally friendly and economical technique for removing salt ions from saline water. In this study, highly efficient hollow carbon nanofiber electrodes for capacitive deionization were prepared using co-axial electrospinning of poly(methyl methacrylate) (core) and poly(acrylonitrile) (shell) polymer solutions, followed by oxidative stabilization and then carbonization. The morphology, pore structure and electrochemical performance were characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM), nitrogen adsorption–desorption isotherms, and cyclic voltammetry, respectively. The synthesized hollow carbon nanofibers had a specific capacitance of 222.3 F g−1, which is almost 4 times higher than the corresponding value for solid carbon nanofibers (63 F g−1). Moreover, the surface area of the hollow nanofibers (186 m2 g−1) was 10 times greater compared to the surface area of solid carbon nanofibers (17.7 m2 g−1). Accordingly, the salt ion electrosorption capacity of the modified carbon nanofibers was greatly enhanced; the hollow nanofibers exhibited an excellent desalination performance (∼86%) and a better cycling ability. These properties are attributed to the hollow structure. Overall, the proposed modification to carbon nanofibers makes them adequate not only for use as promising electrodes for the CDI process but also for any application requiring carbonaceous materials with a high specific surface area.

 Please wait while we load your content...
Please wait while we load your content...