Diastereoselective recognition of α-mannoside by hemicryptophane receptors†
Abstract
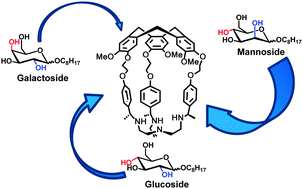
Four new enantiopure hemicryptophanes were synthesized and their absolute configuration was determined from experimental and calculated ECD spectra. Complexation properties of these receptors were studied toward six carbohydrate stereoisomers derived from glucose, galactose and mannose. All the receptors showed a better affinity for α-mannoside with association constants up to 2.5 × 103 M−1. One of the receptor can complex almost exclusively α-mannoside facing to α-galactoside.

 Please wait while we load your content...
Please wait while we load your content...