Incidence of the melting-degradation process of vitamin C on the determination of the phase diagram with acetaminophen enhanced by high performance liquid chromatography tools
Abstract
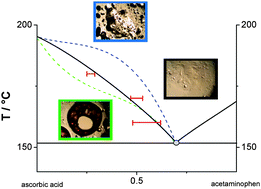
Compounds that degrade upon melting, such as vitamin C, are not convenient for temperature-composition phase diagram survey. To bypass such a disadvantage, faster scan rates may be used to heat the samples. However, in that case, the thermodynamic solid–liquid equilibrium cannot be reached as the present study demonstrates regarding the vitamin C–acetaminophen phase diagram determination. In this work, high performance liquid chromatography has therefore been used as a complementary tool with differential scanning calorimetry to determine the appropriate liquidus point for various mole fractions of vitamin C–acetaminophen. Because a classical thermodynamic approach is not sufficient for such a study, the analytical experiments that we have conducted provide useful data, allowing us to determine the non-degraded vitamin C quantity in the vitamin C–acetaminophen mixtures treated at a given temperature and annealing time.

 Please wait while we load your content...
Please wait while we load your content...