Thermodynamic data for Pb2+ and Zn2+ sequestration by biologically important S-donor ligands, at different temperatures and ionic strengths
Abstract
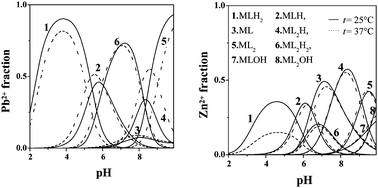
Thermodynamic parameters for the interactions of cysteine (cys) and penicillamine (psh) with Pb(II) and glutathione (gsh) with Pb(II) and Zn(II) were determined in NaNO3 or NaCl aqueous solution by potentiometry, at different ionic strengths (0 < I ≤ 1 mol L−1) and temperatures (15 ≤ t ≤ 45 °C). For Pb2+ systems, the formation of PbL, PbLH, PbLH2 and PbL2 species was found, together with PbLOH for cys and psh, and PbL2H and PbL2H2 for gsh. The speciation models for Pb2+–psh and –gsh systems were confirmed by UV spectrophotometric measurements. For the Zn2+–gsh system, a more complex speciation model was obtained with the formation of ZnL, ZnLH, ZnLH2, ZnL2, ZnL2H, ZnL2H2, ZnLOH and ZnL2OH species. From the dependence of formation constants on temperature, rough ΔH values were evaluated: the main contribution to the complexation free energy is entropic in nature, with small enthalpic values. Moreover, from the dependence on ionic strength, formation constants extrapolated at I = 0 mol L−1 were obtained. The sequestering ability of the ligands towards Pb2+ and Zn2+ was evaluated by determining the pL0.5, i.e. the −log of the concentration of the ligand able to complex half of the metal ion fraction. All these ligands show good sequestering ability. For Pb2+–cys, –psh and –gsh systems, pL0.5 reaches the value of 8.2, 9.0 and 5.9, respectively, at pH = 7, I = 0.1 mol L−1 and t = 25 °C. Under the same experimental conditions, the sequestering ability of gsh towards Zn2+ is lower, with pL0.5 = 4.1.

 Please wait while we load your content...
Please wait while we load your content...