One-pot and highly regio-selective 1,3-dipole cycloaddition of azomethine ylide generated in situ to tetraethyl vinylidenebisphosphonate (VBP) catalyzed by cerium(iv) oxide†
Abstract
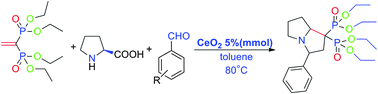
VBP reacts smoothly with L-proline and benzaldehyde derivatives at 80 °C in toluene catalyzed by cerium(IV) oxide (CeO2) to give the corresponding heterocyclic bisphosphonates in moderate yields (30–70%). A high regio-selectivity of the 1,3-dipole cycloaddition was observed. The structures of the targeted molecules are characterized by NMR (such as COSY, HSQC, and HMBC), IR and MS.

 Please wait while we load your content...
Please wait while we load your content...