Exploring the influence of cationic skeletons on the arrangement of isolated BO3 groups based on RbMgBO3, CsZn4(BO3)3 and Cs4Mg4(BO3)4†
Abstract
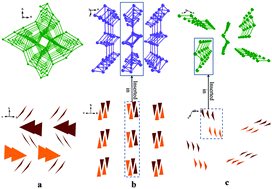
The structures of three borates with isolated BO3 groups, RbMgBO3, CsZn4(BO3)3 and Cs4Mg4(BO3)4, are determined from single crystal diffraction data for the first time. The introduction of small cations (Mg2+ and Zn2+) with strong bonding can prevent the formation of the B–O network effectively, and is beneficial to obtain the isolated BO3 groups. Moreover, the cationic skeletons composed of small cations significantly affect the arrangement of isolated BO3 groups. Thermal analysis, infrared and UV-Vis-NIR diffuse reflectance spectroscopy, and electronic band structure calculations were performed for the reported materials.

 Please wait while we load your content...
Please wait while we load your content...