Rhodamine-based molecular clips for highly selective recognition of Al3+ ions: synthesis, crystal structure and spectroscopic properties†
Abstract
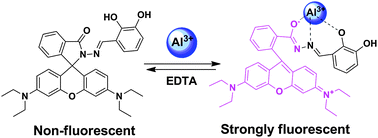
A novel fluorescent chemosensor based on a rhodamine derivative (L) was designed, synthesized, and used as a selective Al+3 ion sensor. Upon addition of Al3+ to an aqueous-acetonitrile solution of L, the development of a strong fluorescence signal by a chelation-enhanced fluorescence (CHEF) process was observed with an attractive glowing orange emission. This sensor shows high selectivity towards Al3+ ions in the presence of other competing metal ions. The fluorescence quantum yield of L–Al3+ (Φf = 0.30) was found to be very high compared to the bare ligand. The limit of detection (LOD) of Al3+ ions was calculated to be 2 × 10−8 M by fluorescence titration. The 1 : 1 binding stoichiometry of the metal complex was established by combined UV-vis, fluorescence and TOF-MS spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...