Uptake mechanisms for inorganic iron and ferric citrate in Trichodesmium erythraeum IMS101
Abstract
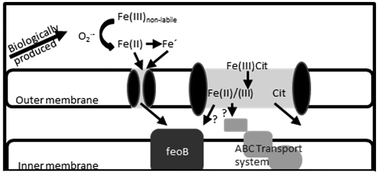
Growth of the prevalent marine organism Trichodesmium can be limited by iron in natural and laboratory settings. This study investigated the iron uptake mechanisms that the model organism T. erythraeum IMS101 uses to acquire iron from inorganic iron and iron associated with the weak ligand complex, ferric citrate. IMS101 was observed to employ two different iron uptake mechanisms: superoxide-mediated reduction of inorganic iron in the surrounding milieu and a superoxide-independent uptake system for ferric citrate complexes. While the detailed pathway of ferric citrate utilization remains to be elucidated, transport of iron from this complex appears to involve reduction and/or exchange of the iron out of the complex prior to uptake, either at the outer membrane of the cell or within the periplasmic space. Various iron uptake strategies may allow Trichodesmium to effectively scavenge iron in oligotrophic ocean environments.
- This article is part of the themed collection: Metals in marine biochemistry

 Please wait while we load your content...
Please wait while we load your content...