Synthesis, enantiomeric separation and docking studies of spiropiperidine analogues as ligands of the nociceptin/orphanin FQ receptor†
Abstract
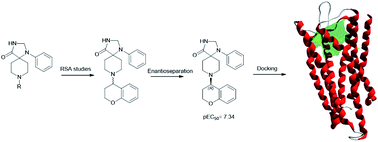
A series of triazospirodecanone derivatives were synthesized as potential NOP ligands. 8-(Chroman-4-yl)-1-phenyl-1,3,8-triazaspiro[4.5]decan-4-one (4) and its 5-fluoro analogue (18) proved to be active as agonists with EC50 values in the submicromolar range. Single enantiomers of compound 4 were separated and tested as NOP agonists; the eutomer R-(+)-4 showed a pEC50 of 7.34. Finally docking studies were performed on the NOP receptor to identify the most significant stereospecific interactions.

 Please wait while we load your content...
Please wait while we load your content...