3,4,2′-Trimethoxy-trans-stilbene – a potent CYP1B1 inhibitor†
Abstract
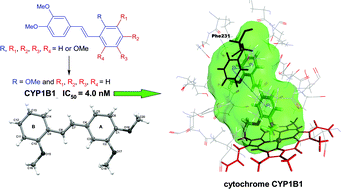
A novel series of methoxy-trans-stilbenes with 3,4-dimethoxy motifs was designed and synthesized. The inhibitory potency of 3,4-dimethoxystilbene derivatives against cytochrome P450 isozymes CYP1A1, CYP1B1 and CYP1A2 was evaluated. 3,4,2′-Trimethoxy-trans-stilbene (3,4,2′-TMS) exhibited extremely potent inhibitory action against CYP1B1 activity with an IC50 of 0.004 μM. 3,4,2′-TMS exhibited 90-fold selectivity for CYP1B1 over CYP1A1 and 830-fold selectivity for CYP1B1 over CYP1A2. However, 3,4,2′,4′-tetramethoxy-trans-stilbene appeared to be the most selective inhibitor of both CYP1B1 and CYP1A1 showing very low affinity toward CYP1A2. Complementary experimental studies and computational methods were used to explain what structural determinants decide the specific affinity of stilbene derivatives to CYP1A2 and CYP1B1 binding sites.

 Please wait while we load your content...
Please wait while we load your content...