Osthol and curcumin as inhibitors of human Pgp and multidrug efflux pumps of Staphylococcus aureus: reversing the resistance against frontline antibacterial drugs†‡
Abstract
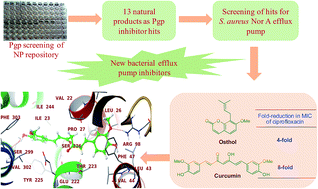
The in-house IIIM natural product repository of 302 small molecules was screened for their ability to inhibit P-glycoprotein (Pgp) in Pgp-overexpressing human adenocarcinoma LS-180 cells. The screening has identified 13 natural products displaying significant Pgp-inhibition activity, which include praeruptorin B, curcumin, imperatorin, osthol, 5,7-diacetoxy-8-(3-methyl-2-butenyl)-coumarin, 5,7-dihydroxy-8-(3-methyl-2-butenyl) coumarin, pongamol, phellopterin, tangeretin, 3-(2-methyl but-3-en-2-yl) xanthyletin, 7-demethyl osthol, allorottlerin and tetrahydroangeolide. These natural products were then screened for their effects on bacterial efflux pump inhibition activity against NorA (Staphylococcus aureus), MdeA (S. aureus Mupr-1), TetK (S. aureus SA-K2192), and MsrA (S. aureus SA-K2191) efflux pumps. Curcumin and osthol showed significant inhibition of the S. aureus NorA efflux pump with 8- and 4-fold reductions in the MIC of ciprofloxacin at 25 μM. The molecular docking studies of curcumin and osthol with the human Pgp and S. aureus NorA efflux pump identified plausible binding modes and binding sites for these natural products.

 Please wait while we load your content...
Please wait while we load your content...