Antisense oligonucleotides: modifications and clinical trials
Abstract
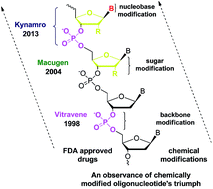
There has been an upsurge in the number of clinical trials involving chemically modified oligonucleotide-based drug candidates after the FDA approval of Vitravene, Macugen, and recently, Kynamro. Over the years, different types of backbone, nucleobase and/or sugar-modified oligonucleotides have been synthesized because natural DNA/RNA based oligonucleotides pose some limitations, such as poor binding affinity, low degree of nuclease resistance, affecting their direct use in antisense therapeutics. In this review article, we discuss in detail different modifications of nucleosides/oligonucleotides along with the related clinical trials, which demonstrated their potential as drug candidates for antisense and related nucleic acid based therapeutics.

 Please wait while we load your content...
Please wait while we load your content...