Conformational analysis of peramivir reveals critical differences between free and enzyme-bound states†
Abstract
Peramivir is a potent inhibitor of influenza neuraminidase, and is used clinically to treat influenza infections. The substantial potency of peramivir for its target suggests that similar structures might be useful as lead compounds for designing inhibitors of related viral, mammalian, or bacterial neuraminidases. At the same time, the large number of rotatable bonds in peramivir's structure led us to consider the conformational flexibility for the drug, since a more flexible scaffold might be a disadvantage in cases where isoenzyme selectivity is required. An examination of previously published X-ray data for the free and bound states of the drug, together with solution-phase NMR, conformational analysis, and DFT calculations leads us to conclude that peramivir undergoes a substantial conformational shift upon binding to the neuraminidase active site. Peramivir's previously unrecognized conformational flexibility may be a liability for peramivir itself, or for future applications of the underlying cyclopentane scaffold. Our analysis finds a consensus among enzyme-bound conformations of the inhibitor, and suggests that favoring this conformation could be used to develop inhibitors with greater potency or isoform selectivity.
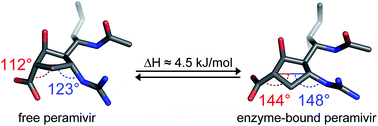

 Please wait while we load your content...
Please wait while we load your content...