Inhibitors of the human neuraminidase enzymes
Abstract
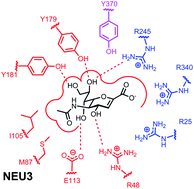
The human neuraminidase enzymes (hNEU) are a family of four isoenzymes that hydrolyze sialosides, including gangliosides and glycoproteins. These enzymes are proposed to play roles in several important signaling pathways and are implicated in diseases such as diabetes and cancer. Despite their importance, only a limited number of studies have sought to identify potent inhibitors for these enzymes. This review summarizes the substrate specificity and known inhibitors of the hNEU isoenzymes, as well as the emerging work on development of isoenzyme-specific inhibitors.

 Please wait while we load your content...
Please wait while we load your content...