Identification and optimisation of 3,3-dimethyl-azetidin-2-ones as potent and selective inhibitors of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1)†
Abstract
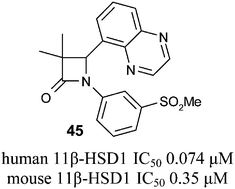
3,3-Di-methyl-azetidin-2-ones were identified as potent and selective 11β-HSD1 inhibitors against the human and mouse forms of the enzyme. Structure guided optimisation of LLE was conducted, utilising a key polar interaction and identifying stereochemical preference for the 4S isomer. Metabolic stability was improved to afford oral exposure, providing tool compounds suitable for pre-clinical evaluation.

 Please wait while we load your content...
Please wait while we load your content...