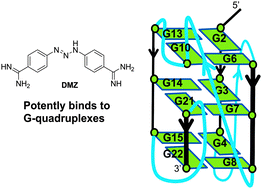
Diminazene or berenil, a classic duplex minor groove binder, binds to G-quadruplexes with low nanomolar dissociation constants and the amidine groups are also critical for G-quadruplex binding†
Abstract
G-quadruplexes have shown great promise as chemotherapeutic targets, probably by inhibiting telomere elongation or downregulating oncogene expression. There have been many G-quadruplex ligands developed over the years but only a few have drug-like properties. Consequently only a few G-quadruplex ligands have entered clinical trials as cancer chemotherapeutic agents. The DNA minor groove ligand, berenil (diminazene aceturate or DMZ), is used to treat animal trypanosomiasis and hence its toxicological profile is already known, making it an ideal platform to engineer into new therapeutics. Herein, using a plethora of biophysical methods including UV, NMR, MS and ITC, we show that DMZ binds to several G-quadruplexes with a Kd of ∼1 nM. This is one of the strongest G-quadruplex binding affinities reported to date and is 103 tighter than the berenil affinity for an AT-rich duplex DNA. Structure–activity-relationship studies demonstrate that the two amidine groups on DMZ are important for binding to both G-quadruplex and duplex DNA. This work reveals that DMZ or berenil is not as selective for AT-rich duplexes as originally thought and that some of its biological effects could be manifested through G-quadruplex binding. The DMZ scaffold represents a good starting point to develop new G-quadruplex ligands for cancer cell targeting.

 Please wait while we load your content...
Please wait while we load your content...