Phosphorylation of Ser8 promotes zinc-induced dimerization of the amyloid-β metal-binding domain†
Abstract
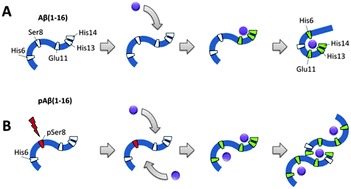
Zinc-induced aggregation of the amyloid-β peptide (Aβ) is a hallmark molecular feature of Alzheimer's disease (AD). Recently it was shown that phosphorylation of Aβ at Ser8 promotes the formation of toxic aggregates. In this work, we have studied the impact of Ser8 phosphorylation on the mode of zinc interaction with the Aβ metal-binding domain 1–16 using isothermal titration calorimetry, electrospray ionization mass spectrometry and NMR spectroscopy. We have discovered a novel zinc binding site (6HDpS8) in the phosphorylated peptide, in which the zinc ion is coordinated by the imidazole ring of His6, the phosphate group attached to Ser8 and a backbone carbonyl group of His6 or Asp7. Interaction of the zinc ion with this site involves His6, thereby withdrawing it from the interaction pattern observed in the non-modified peptide. This event was found to stimulate dimerization of peptide chains through the 11EVHH14 site, where the zinc ion is coordinated by the two pairs of Glu11 and His14 in the two peptide subunits. The proposed molecular mechanism of zinc-induced dimerization could contribute to the understanding of initiation of pathological Aβ aggregation, and the 11EVHH14 tetrapeptide can be considered as a promising drug target for the prevention of amyloidogenesis.

 Please wait while we load your content...
Please wait while we load your content...