Paired single cell co-culture microenvironments isolated by two-phase flow with continuous nutrient renewal†
Abstract
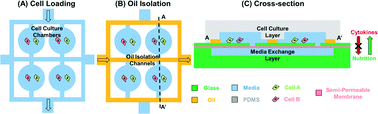
Cancer–stromal cell interactions are a critical process in tumorigenesis. Conventional dish-based assays, which simply mix two cell types, have limitations in three aspects: 1) limited control of the cell microenvironment; 2) inability to study cell behavior in a single-cell manner; and 3) have difficulties in characterizing single cell behavior within a highly heterogeneous cell population (e.g. tumor). An innovative use of microfluidic technology is for improving the spatial resolution for single cell assays. However, it is challenging to isolate the paired interacting cells while maintaining nutrient renewal. In this work, two-phase flow was used as a simple isolation method, separating the microenvironment of each individual chamber. As nutrients in an isolated chamber are consumed by cells, media exchange is required. To connect the cell culture chamber to the media exchange layer, we demonstrated a 3D microsystem integration technique using vertical connections fabricated by deep reactive-ion etching (DRIE). Compared to previous approaches, the presented process allows area reduction of vertical connections by an order of magnitude, enabling compact 3D integration. A semi-permeable membrane was sandwiched between the cell culture layer and the media exchange layer. The selectivity of the semi-permeable membrane results in the retention of the signaling proteins within the chamber while allowing free diffusion of nutrients (e.g., glucose and amino acids). Thus, paracrine signals are accumulated inside the chamber without cross-talk between cells in other chambers. Utilizing these innovations, we co-cultured UM-SCC-1 (head and neck squamous cell carcinoma) cells and endothelial cells to simulate tumor proliferation enhancement in the vascular endothelial niche.

 Please wait while we load your content...
Please wait while we load your content...