DVD technology-based molecular diagnosis platform: quantitative pregnancy test on a disc†
Abstract
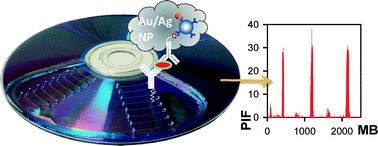
A diagnosis platform based entirely on DVD technology was developed for on-site quantitation of molecular analytes of interest, e.g., human chorionic gonadotropin (hCG) in urine samples (“quantitative pregnancy test on a disc”). An hCG-specific monoclonal antibody-binding assay prepared on a regular DVD-R was labeled with nanogold–streptavidin conjugates for signal enhancement with a customized silver-staining protocol. An unmodified, conventional computer optical drive was used for assay reading, and free disc-quality analysis software for data processing. The performance (sensitivity and selectivity) of this DVD assay is comparable to that of well-established colorimetric methods (determination of optical darkness ratios) and standard enzyme-linked immunosorbent assays (ELISA). As validated by examining its linear correlation with the ELISA results on the same set of samples, the DVD assay promises to be a low-cost, multiplex, point-of-care (POC) diagnostic tool for physicians and even for individuals at home, producing prompt results.

 Please wait while we load your content...
Please wait while we load your content...