Amidation of phenol derivatives: a direct synthesis of paracetamol (acetaminophen) from hydroquinone†
Abstract
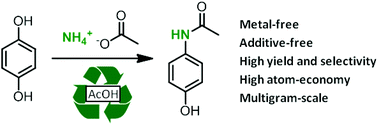
A direct synthesis of paracetamol (acetaminophen) from hydroquinone has been developed using ammonium acetate as an amidating agent. The reaction proceeds in acetic acid at elevated temperatures without any metallic catalyst. Under these conditions, paracetamol was obtained with high yield and selectivity (>95%). The reaction has also been carried out on the multi-gram scale (44 g of hydroquinone) and a potential process has been proposed based on the recycling of the solvent and by-products. This amidation protocol has also been extended to other phenol derivatives.

 Please wait while we load your content...
Please wait while we load your content...