Fixation and recycling of nitrogen monoxide through carbonitrosation reactions†
Abstract
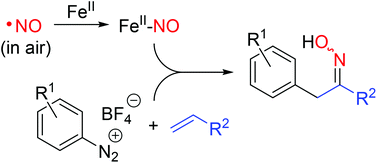
The removal of nitrogen monoxide from gas streams through complexation to iron(II) ions in aqueous dimethylsulfoxide can be combined with a new variant of the Meerwein arylation, which incorporates the previously complexed NO into organic compounds to give oximes as final products. The first step of this two-step process has been evaluated regarding the effectiveness of the NO absorption and the sensitivity of the aqueous iron(II)–DMSO solution towards oxygen from air, in both cases in comparison with the known BioDeNOx process. The subsequent Meerwein arylation, which was designed with the intention to make use of nitrogen monoxide as the simplest nitrogen-centered radical scavenger, is shown to tolerate an exceptionally broad spectrum of substituents on the aromatic core of the diazonium salts including electron-donating as well as electron-withdrawing substituents. Under simple conditions the resulting oximes can be converted to racemic amino acid esters.

 Please wait while we load your content...
Please wait while we load your content...