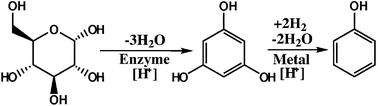
Utilization of renewable sugars from biomass by a hybrid chemical process produces highly oxygenated aromatic compounds, such as phloroglucinol, which require catalytic reduction for desirable aromatic products. Aqueous phase hydrodeoxygenation of phloroglucinol on carbon-supported platinum produces resorcinol, phenol, cyclohexanol, cyclohexanone, and 1,3-cyclohexanediol by combinations of carbon–oxygen bond cleavage and carbon–carbon double bond hydrogenation. Carbon–carbon σ-bond cleavage was not observed. Hydrodeoxygenation was the primary reaction of phloroglucinol, leading to the production of resorcinol in the overall rate-limiting reaction, with an activation energy barrier of Ea = 117 kJ mol−1. Subsequent reactions of resorcinol produced 1,3-cyclohexanediol and phenol with similar energy barriers, Ea = 46 and Ea = 54 kJ mol−1, respectively. Further hydrogenation of phenol (Ea = 42 kJ mol−1) occurs through the intermediate, cyclohexanone, which is further reduced (Ea = 14 kJ mol−1) to the dominant product, cyclohexanol.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...