Selective hydrogenation of 5-hydroxymethylfurfural to 2,5-bis-(hydroxymethyl)furan using Pt/MCM-41 in an aqueous medium: a simple approach†
Abstract
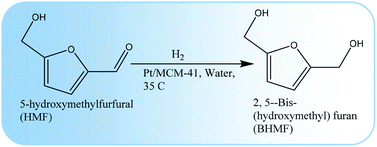
The hydrogenation of HMF has been conducted in a neutral aqueous medium. Without any additive, HMF was hydrogenated to 2,5-bis-(hydroxymethyl)furan (BHMF) with complete conversion and selectivity (98.9%) using Pt/MCM-41 as catalyst. A very low temperature of 35 °C and 0.8 MPa of hydrogen pressure was used to accomplish the highest selectivity of BHMF within a reaction time of 2 h. Different reaction parameters such as reaction time, hydrogen pressure and the amount of water was optimized to achieve the highest catalytic activity. In particular, the presence or absence of water and the amount of water played an important role to determine the conversion and product distribution of the reaction. For instance, in the absence of water or a large excess of water, the selectivity of BHMF was decreased. In addition, instead of water the influence of three different groups of organic solvent were also explored to obtain BHMF under the studied reaction conditions. It has been observed that the studied organic solvents strongly influenced the catalytic performance, such as solvents with a negative δ value, which followed a clear trend with the substrate conversion, whereas no impact was observed for solvents with a positive δ value. Catalyst recycling experiments revealed that the catalyst could be recycled several times without any significant loss of catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...