Rational investigations in the ring opening of cyclic carbonates by amines†
Abstract
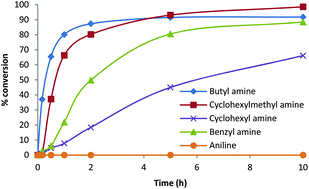
Non-isocyanate polyurethanes (NIPUs) constitute a promising alternative for more classical polyurethanes (PUs) as they may display mechanical properties that can match those of PUs and their synthesis does not involve the use of toxic isocyanates. Yet, because of the lower reactivity of carbonates versus isocyanates, the synthesis of NIPUs is not straightforward and generally requires the use of a catalyst. Recently, several groups have reported on the use of different ranges of catalysts for promoting the nucleophilic attack of the amine on the carbonate. However, many of these studies involve the use of highly reactive amine and/or carbonate proscribing a complete panorama of the potentialities of the reaction. Herein, we propose a rational study of the catalyzed aminolysis of four representative cyclic carbonates that reveals that the thiourea organocatalyst 1 outperforms in many aspects, classical inorganic or other organic catalysts.

 Please wait while we load your content...
Please wait while we load your content...