Oxidation of tetrahydrofuran to butyrolactone catalyzed by iron-containing clay
Abstract
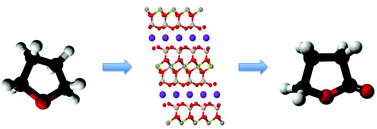
Thermally treated iron-containing clay was used as a greener oxidation catalyst for the conversion of tetrahydrofuran (THF) to butyrolactone (BTL). Mild liquid phase reactions were tested at 50–66 °C using hydrogen peroxide (H2O2) as an oxidizing agent. XRD, TGA, ESR, DR-UV, and FTIR revealed the dislodged iron oxide species formed by treating at ≥500 °C. Formation of active oxidizing species on the surface occurs on contact the dislodged Fe(III) oxide with H2O2. Such active species can promote the oxidation of THF, giving high yield and selectivity of BTL, whereas the iron-containing clay treated at lower temperatures (<500 °C) perform Fenton-like oxidation with lower THF conversions and non-selective products. 2-Hydroxytetrahydrofuran (THF-2-ol) was primarily produced and further oxidized to BTL with a small amount of 4-hydroxybutyric acid as a minor product. Minimal H2O2/THF ratio of 1.0 is sufficient for the production of BTL. Deactivation can be observed presumably due to deposition of the products despite slight leaching of the active iron species.

 Please wait while we load your content...
Please wait while we load your content...