Bare magnetic nanoparticles: sustainable synthesis and applications in catalytic organic transformations
Abstract
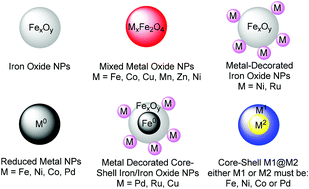
Magnetic nanoparticles have become increasingly attractive in the field of catalysis over the last decade as they combine interesting reactivity with an easy, economical and environmentally benign mode of recovery. Early strategies focused on the use of such nanoparticles as a vehicle for supporting other catalytic nanomaterials or molecules to facilitate recovery. More recently, research has shown that bare magnetic nanoparticles may serve the dual role of a catalyst and a magnetically recoverable entity. At the same time, emerging sustainability concepts emphasize the utility of earth abundant and less toxic resources, especially iron. Herein, we review the recent progress made in the assembly of such systems and their direct application in catalysis. Examples of such bare nanoparticles include iron oxide (Fe2O3 and Fe3O4), metal ferrites (MFe2O4, M = Cu, Co and Ni), Fe(0), Co(0), Ni(0), and multi-component nanoparticles. Features such as reactivity, recoverability and leaching are discussed in a critical fashion.

 Please wait while we load your content...
Please wait while we load your content...