Efficient ligand-free Hiyama cross-coupling reaction catalyzed by functionalized SBA-15-supported Pd nanoparticles†
Abstract
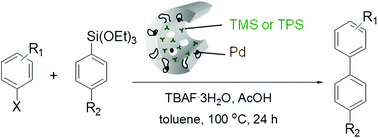
A ligand-free Hiyama cross-coupling reaction catalyzed by functionalized SBA-15-supported Pd catalysts has been developed. The catalysts were prepared by depositing Pd nanoparticles preferentially in the micropores of the SBA-15 with hydrophobic trimethylsilyl or triphenylsilyl groups grafted on the mesopores. The nanocomposite catalysts showed excellent activities for the cross-coupling between various aryltriethoxysilanes and aryl halides under relatively mild (at 100 °C in air) reaction conditions. Moreover, the hydrophobic functionalization rendered the catalysts reusable without showing significant activity loss. The decreased activity after successive catalytic runs was attributed to a low level of Pd-leaching and a gradual collapse of mesopores of host silica. The cross-coupling protocol with the designed catalysts would be practical for use as an economical synthetic method for the construction of biphenyl derivatives.

 Please wait while we load your content...
Please wait while we load your content...