Efficient and selective copper-catalyzed organic solvent-free and biphasic oxidation of aromatic gem-disubstituted alkenes to carbonyl compounds by tert-butyl hydroperoxide at room temperature†
Abstract
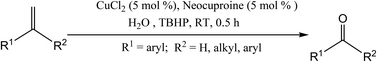
Copper-catalyzed alkene oxidation to carbonyl compounds by tert-butyl hydroperoxide (TBHP) under organic solvent-free and biphasic conditions at room temperature is selective for the aromatic gem-disubstituted alkenes. Enhanced reactivity was observed in the presence of 2,9-dimethyl-1,10-phenanthroline (neocuproine). The reaction is economically attractive because the yield is high, and separation of products and recycling of the catalyst are easy.

 Please wait while we load your content...
Please wait while we load your content...