A continuous process for glyoxal valorisation using tailored Lewis-acid zeolite catalysts†
Abstract
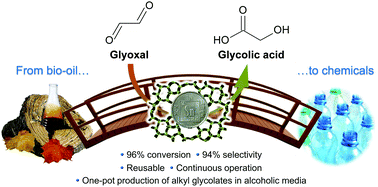
The aqueous-phase heterogeneously catalysed isomerisation of bio-oil derived glyoxal is herein introduced as a novel route for the sustainable production of glycolic acid. While commercial ultra-stable Y zeolites displayed only moderate performance, their evaluation enabled us to highlight the crucial role of Lewis acidity in the reaction. Gallium incorporation into these zeolites boosted the glycolic acid yield, although the best catalytic results were obtained over tin-containing MFI-type zeolites, reaching 91% yield of the desired product at full conversion. These materials comprised hydrothermally-synthesised Sn-MFI as well as a novel catalyst obtained by the introduction of tin into silicalite-1 by means of a simpler and more scalable method, i.e. alkaline-assisted metallation. In-depth spectroscopic characterisation of these systems uncovered a substantial similarity of the tin centres obtained by the top-down and bottom-up synthetic approaches. NMR spectroscopic studies gave evidence that the reaction follows a 1,2-hydride shift mechanism solely catalysed by Lewis-acid sites. The Sn-MFI analogue could be reused in 5 cycles without the need for intermediate calcination, did not evidence any tin leaching, and demonstrated suitability for utilisation under continuous-flow operation. The tin-based zeolites exhibited remarkable performance also in alcoholic solvents, leading to the one-pot production of relevant alkyl glycolates.

 Please wait while we load your content...
Please wait while we load your content...