Enzymatic halogenation of the phenolic monoterpenes thymol and carvacrol with chloroperoxidase†
Abstract
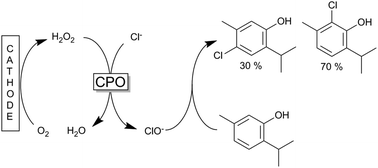
The conversion of the phenolic monoterpenes thymol and carvacrol into antimicrobials by (electro)-chemoenzymatic halogenation was investigated using a chloroperoxidase (CPO) catalyzed process. The CPO catalysed process enables for the first time the biotechnological production of chlorothymol, chlorocarvacrol and bromothymol as well as a dichlorothymol with high conversion rates, total turnover numbers and space time yields of up to 90%, 164 000 and 4.6 mM h−1, respectively.

 Please wait while we load your content...
Please wait while we load your content...